Predictive Ability of LRINEC Score for Lower Extremity Amputation in Moderate to Severe Diabetic Foot Infection
By Abdul Hameed1, Shahid Mehmood Khan1, Muhammad Tawab Khalil2, Ahmed Tariq1, Muhammad Fawad1, Muhammad Ajmal Leghari1Affiliations
doi: 10.29271/jcpsp.2024.04.456ABSTRACT
Objective: To assess the predictive ability of the laboratory risk indicator for necrotising fasciitis (LRINEC) score for lower extremity amputation in patients with moderate to severe diabetic foot infection (DFI).
Study Design: Observational study.
Place and Duration of the Study: Department of General Surgery, Combined Military Hospital, Rawalpindi, Pakistan, from June to September 2023.
Methodology: Patients admitted to the surgical ward with moderate to severe DFI were included by convenience sampling. Patients with severe sepsis, unstable haemodynamics, pressure injuries, and terminal illnesses were excluded. Demographic and clinical data of patients were noted down. LRINEC score was calculated on the day of admission. Final outcome (amputation or otherwise) was recorded on the 30th day the since the day of admission.
Results: Two hundred patients with moderate to severe DFI were included. The median age of patients was 56 years (IQR 49-66 years). The median duration of diabetes was 11 years (IQR 4 - 18.75 years). The median LRINEC score at admission was 6 (IQR 3-9). The majority of the patients (65.5%) had some other medical comorbid besides diabetes. Patients who had amputation due to DFI at 30 days post-admission had higher LRINEC scores on admission as compared to those patients who did not have amputation (Median 8 vs. 2, p <0.001). The cut-off point of LRINEC score ≥6.5 at admission had sensitivity of 74% and specificity of 94% in predicting amputation.
Conclusion: The LRINEC score may be used as an objective scoring system to predict the risk of amputation in patients with moderate to severe DFI in indoor clinical settings.
Key Words: Diabetic foot, LRINEC score, Limb loss, Necrotising fasciitis.
INTRODUCTION
Diabetes mellitus (DM) constitutes a spectrum of metabolic disorders that share hyperglycaemia as its hallmark feature.1 The pandemic of diabetes continues to rise globally. According to the International Diabetes Foundation, 537 million adults aged between 20 and 79 years are living with diabetes globally.2 Diabetes-related lower extremity complications (DRLECs) affect approximately 131 million people around the globe i.e. every 4th person with diabetes has some form of DRLECs. DRLECs occur mainly due to peripheral vascular disease (PVD) and neuropathy.3 Of these, diabetic foot infection (DFI) is the most frequent entity requiring hospitalisation.2
It is the most frequent triggering event leading to lower limb amputation in diabetic patients.4,5 Only 46% of patients with diabetes-related foot ulcer (DFU) report complete healing, while 15% of patients succumb to the disease, and as many as 17% undergo lower extremity amputation within one year.5,6
Different classification systems have been used in the literature to classify DFI according to the severity and extent of infection. International Working Group on Diabetic Foot (IWGDF) Classification, Infectious Diseases Society of America (IDSA) Classification, Wagner’s Classification of Diabetic Foot, PEDIS (Perfusion, Extent, Depth, Infection, Sensation) score and DEPA (Depth of Ulcer, Extent of bacterial colonisation, Phase of ulcer healing, Associated aetiology) score are commonly used in clinical practice. Out of these, IWGDF/IDSA classification for diabetic foot infection is widely accepted. This classification system is easy to implement in clinical settings.2 All these classification systems give information regarding the severity of DFI, but cannot predict the prognosis of disease in terms of risk for amputation.7
LRINEC (The Laboratory Risk Indicator for Necrotising Fasciitis) score is a 13-point score that is used to diagnose necrotising fasciitis.8 It is calculated from six routine laboratory parameters: C-reactive protein (<150mg/L=0; >150mg/L=4), total leukocyte count (<15000/µL=0; 15000-25000/µL=1; >25000/µL=2), haemoglobin (>13.5g/dL=0; 11-13.5g/dL=1; <11g/dL=2), creatinine (≤141 µmol/L=0; >141 µmol/L=2), sodium (≥135mEq/L=0; <135mEq/L=2) and glucose (≤180 mg/dL=0; >180 mg/dL=2).8 This is a validated tool for diagnosing necrotising fasciitis.8 This tool has been used to describe the severity of necrotising fasciitis as well. Multiple risk factors of amputation in patients with DFI have been described in the literature. These include age, duration of diabetes, glycaemic control, smoking, and medical comorbids.9,10 However, the literature on objective scoring system for predicting risk of amputation in patients with DFI is limited. In this study, the authors hypothesised that higher LRINEC score is associated with severity of DFI and this can be used to predict amputation in this patient population. To the authors’ knowledge, LRINEC score has not been used to predict risk of amputation in moderate to severe DFI in Pakistan. This study aimed to provide a cut-off value of LRINEC score for predicting risk of amputation in moderate to severe DFI.
METHODOLOGY
This longitudinal observational study was conducted at the Department of General Surgery, Combined Military Hospital, Rawalpindi, Pakistan, from June to September 2023 after approval from the hospital’s ethical review board. A sample size of 200 participants was calculated using wnarifin sample size calculator with expected sensitivity of LRINEC score 69%, specificity of 52% and prevalence of disease 43%, confidence level 100(1 - α) 95% and expected dropout rate of 4%.11,12
Patients of either gender, with diabetes mellitus (type I and II) aged between 20-79 years who had DFI Grade 3 (moderate) or Grade 4 (severe) as classified by the IWGDF/IDSA classification were included irrespective of history of prior DFI or amputation. Patients were excluded if they had Grade 1 or 2 DFI, severe sepsis or septic shock, uncontrolled hypertension, unstable coronary artery disease, acute limb ischaemia, pressure injuries, uncontrolled seizure disorder, impaired cognition, pregnant females, history of malignancy, pressure ulcers on lower extremities due to stroke or spinal cord injury, causes of skin inflammation other than DFI (venous stasis, thrombosis, thrombophlebitis, dermatitis, trauma, bullous disorders, gout, and fracture) and patients having some other focus of infection besides diabetic foot.
Convenience sampling method was used. All patients admitted with DFI during the study period were screened on the day of admission. Those meeting the inclusion criteria underwent a thorough clinical examination and history by resident surgeon on the same day. Participants were briefed about the nature of the study and informed consent was taken from them. Primary source of data collection was clinical examination and medical records of the patients. Resident surgeons were trained in applying LRINEC score and grading of DFI as per IWGDF classification.
On admission in the surgical ward, demographic characteristics (age, gender) and clinical characteristics (duration of diabetes, glycaemic control medications, medical comorbidities, laterality of involved foot, previous history of DFI, history of DFI resulting in amputation, and present DFI grade) were noted down on data collection forms. Laboratory parameters related to calculation of LRINEC score were recorded from the medical records of patients, and LRINEC score was thus calculated on the day of admission. Patients were followed for 30 days starting from the day of admission. Primary outcome that is presence or absence of amputation (minor or major) was noted down on 30th day. Patient data were de-identified before analysis.
DFI was classified using IWGDF classification. Moderate DFI is defined as erythema extending more than 2 cm from the margin of the wound with or without involvement of deeper structures to skin and subcutaneous tissues (e.g. bone, muscle tendon and joint). However, there are no associated systemic manifestations of infection.2 In severe DFI, there are associated systemic manifestations (≥2 out of these: (i) heart rate more than 90 beats/min (ii) temperature more than 38°C or less than 36°C (iii) respiratory rate more than 20 breaths/min OR PaCO2 less than 4.3 kPa (32 mmHg) (iv) white blood cell count more than 12,000/mm3 OR less than 4000/mm3 or more than 10% immature /band forms) along with any grade of skin or deeper structures involvement.2 Amputation is defined as resection of a segment of a limb through a bone or a joint – in the transverse anatomical plane.13 In context of DRLECs, any amputation proximal to the ankle joint is known as major amputation while resection through or below the ankle is known as minor amputation.2
Statistical package for social sciences version 23 was used to carry out the statistical analysis. Normality of variables was checked using Shapiro-Wilk test. Frequencies and percentages were computed for quantitative variables. For continuous variables, median and interquartile ranges (IQR) were determined. To study statistically significant association between different categorical variables in history and examination (gender, medical comorbid, DFI grade, history of DFI, history of amputation due to DFI) with risk of amputation, Pearson Chi-square test was applied. The authors’ examined the performance of the LRINEC score on predicting amputation risk using receiver operating characteristic curves (ROC). Statistical significance was considered only if p-value was found <0.05.
RESULTS
During the study period, 317 individuals with DFI were hospitalised, out of which 117 were excluded (Figure 1). A total of 200 patients were enrolled in the study. Of these, 117 (58.5%) were men and 83 (41.5%) were women. The median age of the study group was found to be 56 years (Interquartile range (IQR) 49-66 years). Median duration of diabetes was 11 years in the study group (IQR 4 - 18.75 years). At admission, the median LRINEC score was 6 (IQR 3-9). Table I shows the clinical and demographic characteristics of the patients.
 Figure 1: Sample collection
Figure 1: Sample collection
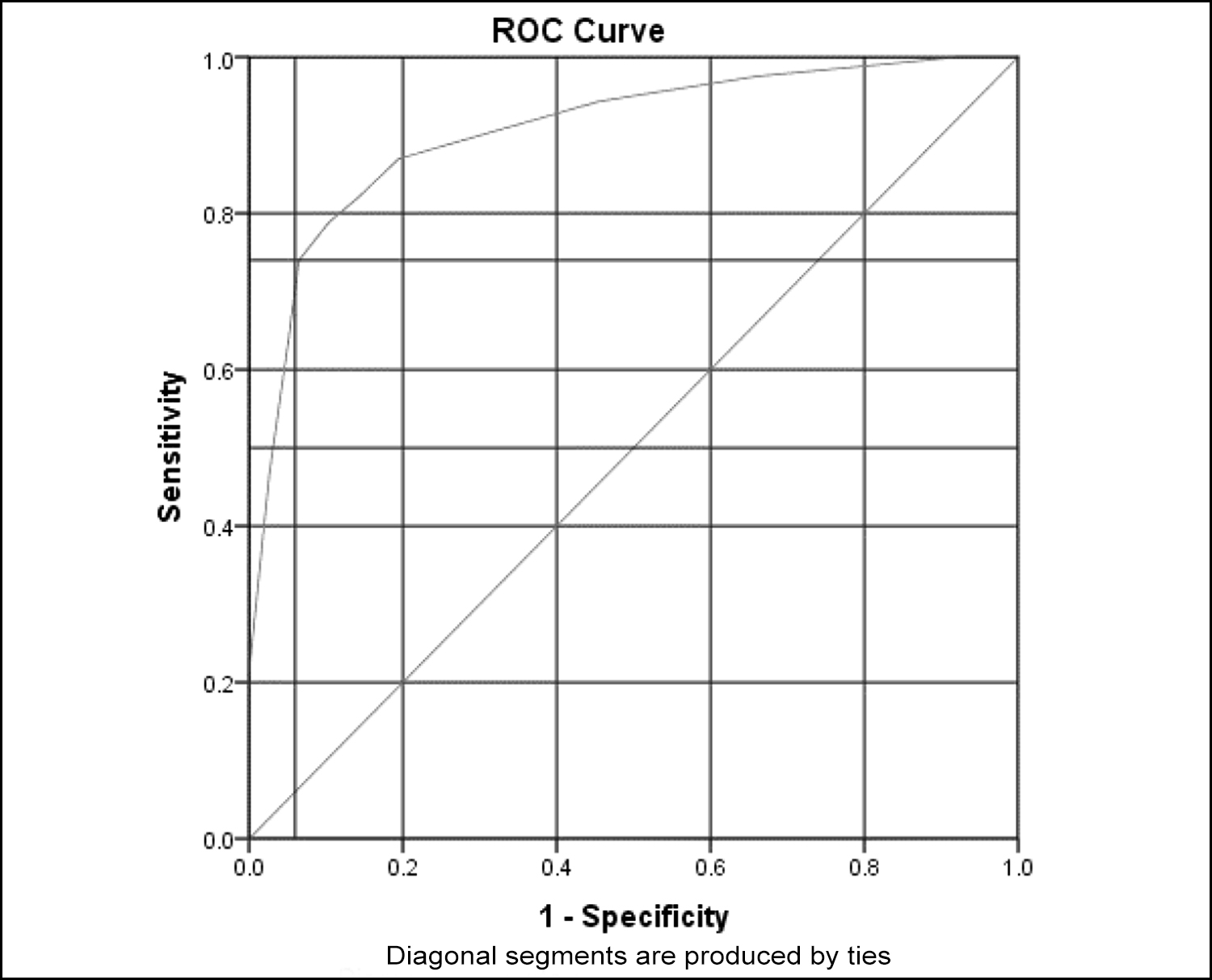 Figure 2: ROC Curve depicting 74% sensitivity and 94% specificity for LRINEC score in predicting amputation.
Figure 2: ROC Curve depicting 74% sensitivity and 94% specificity for LRINEC score in predicting amputation.
Previous history of DFI was present in 123 (61.5%) of patients. Out of these, 60 (30%) patients underwent amputation (major amputation, n = 18 (9%), minor amputations, n = 42 (21%)). Seventy-seven patients (38.5%) had presented with DFI for the first time in their life. Majority of the patients (65.5%) had some other medical comorbid beside diabetes.
Prior to current admission, 47 (23.5%) patients underwent debridement for the current DFI in the past 30 days. Gender of the patient was not associated with the final outcome (p- value 0.243). Previous history of DFI was associated with amputation (p <0.001). Similarly, past history of amputation due to DFI was also associated with risk for amputation in future (p <0.001). Presence of medical comorbid mentioned in Table I, was also associated with the risk of amputation in patients with DFI (p <0.001). Finally, IWGDF grade of DFI was also associated with the outcome (p <0.001) as shown in Table II.
Patients with grade 3 or moderate DFI had lower LRINEC score as compared to patients with grade 4 or severe DFI (Median 3 vs. 9, p <0.001). All patients who had amputation due to DFI at 30 days post-admission had higher LRINEC scores on admission as compared to those patients who did not have amputation (Median 8 vs. 2, p <0.001). The area under the curve of ROC is 0.906 (95% Confidence Interval 0.86 – 0.94, p <0.001) with cut-off value of ≥6.5 in predicting amputation in moderate to severe DFI patients (Figure 2). This cut-off point of LRINEC score had 74% sensitivity and 94% specificity.
Table I: Clinical and demographic characteristics of participants.
|
Variables |
Frequency (N) |
Percentage (%) |
|
Management of diabetes |
||
|
Oral hypoglycaemics |
76 |
38 |
|
Insulin |
124 |
62 |
|
Involved lower extremity |
|
|
|
Right |
111 |
55.5 |
|
Left |
64 |
32 |
|
Bilateral |
25 |
12.5 |
|
IWGDF grade |
||
|
Moderate |
118 |
59 |
|
Severe |
82 |
41 |
|
Previous history of DFI |
|
|
|
Yes |
123 |
61.5 |
|
No |
77 |
39.5 |
|
Comorbidities |
||
|
Hypertension (HTN) |
30 |
15 |
|
Ischaemic heart disease (IHD) |
24 |
12 |
|
HTN and IHD |
41 |
20.5 |
|
Others* |
36 |
18 |
|
None |
69 |
34.5 |
|
Final outcome at 30 days after admission |
||
|
Amputation |
123 |
61.5 |
|
Resolution of DFI with |
77 |
38.5 |
|
*Others - Chronic liver disease, COPD, CKD, dyslipidemia and gastroesophe-geal reflux disease. **Debridement, dressings and antibiotics. |
||
Table II: Association of clinical parameters with amputation.
|
Parameter |
Amputation (Frequency) |
No Amputation (Frequency) |
p-value* |
|
Gender |
|||
|
Male |
68 (34%) |
49 (24.5%) |
0.243 |
|
Female |
55 (25.5%) |
28 (14%) |
|
|
History of DFI |
|||
|
Yes |
106 (53%) |
17 (8.5%) |
<0.001 |
|
No |
17 (8.5%) |
60 (30%) |
|
|
History of amputation due to DFI |
|||
|
Yes |
57 (28.5%) |
3 (1.5%) |
<0.001 |
|
No |
66 (33%) |
74 (37%) |
|
|
Presence of medical comorbids |
|||
|
Yes |
102 (52%) |
29 (14.5%) |
<0.001 |
|
No |
21 (10.5%) |
48 (24%) |
|
|
IWGDF grade |
|||
|
Grade 3 (moderate) |
47 (23.5%) |
71 (35.5%) |
<0.001 |
|
Grade 4 (severe) |
76 (38%) |
6 (3%) |
|
|
*Calculated with Pearson Chi-square test. A p-value of <0.05 is significant. |
|||
DISCUSSION
This study showed that LRINEC score has good diagnostic accuracy in predicting amputation in patients with moderate to severe DFI at a cut-off point of ≥6.5. As LRINEC score is calculated in round figures, a score of ≥7 should be considered cut-off for practical purposes. Demirdal et al. reported a relatively modest diagnostic accuracy of LRINEC score at cut-off value ≥5 in predicting lower extremity amputation in patient with DFI with sensitivity of 69.1% and specificity of 52.3%.14 They included all patients with any grade of DFI to determine the cut off point for LRINEC score which may have resulted in a lower cut-off point as compared to this study.14 Since this study only included patients with moderate to severe DFI, who had higher LRINEC scores to start with, this may be responsible for the higher cut-off point of 6.5, as compared to the previous study.
LRINEC score has proved its value as a diagnostic tool in differentiating necrotising fasciitis from other skin and soft tissue infections.15 Wong et al. reported that LRINEC score had high positive and negative predictive values (92.0% and 96.0%, respectively) and that a score of ≥8 was associated with a higher risk of necrotising soft tissue infection.16 However, subsequent studies demonstrated lower predictive values.17,18 Current consensus is that the LRINEC score is beneficial in identifying high-risk patients among those with necrotising fasciitis rather than diagnosing necrotising fasciitis itself.16,19
In this study, LRINEC score was considerably higher in patients with severe DFI as compared to moderate DFI (median 9 vs. 3) which shows that the severity of DFI is linked to a higher LRINEC score. Furthermore, patients who underwent amputation due to DFI had higher LRINEC scores as compared to those who did not have amputation (median 8 vs. 2). This points out the possible utility of LRINEC score in predicting the severity of DFI and predicting the future course of the disease rather than establishing the diagnosis of DFI. However, further robust studies are needed to test this causal relation.
Besides laboratory parameters, clinical parameters like previous history of DFI or history of amputation due to DFI, moderate to severe DFI and medical comorbid besides diabetes (HTN, IHD and others) are independent risk factors leading to increased amputation risk in people with DFI.20 This is consistent with previous studies in the literature.3,20 Henceforth, the authors suggest that patients with above mentioned clinical profile constitute a high-risk population, and DFI in these patients should be managed more vigilantly.
This study was not without limitations. First of all, lack of a control group and the short duration of follow-up may affect generalisability of the study. This was a single-centeric study. Certain confounding factors like smoking, peripheral vascular disease, and neuropathy may affect the final outcomes which were not addressed in this study. Only patients with moderate to severe DFI were included in this study, this can also restrict the generalisation of results of this study to the population of patients with DFI. However, the authors were able to provide a cut-off value of the LRINEC score in predicting amputation in a specific population of moderate to severe DFI in this setting. Future studies may focus on these areas to further elucidate the diagnostic accuracy of LRINEC scores in predicting amputation in patients with DFI.
CONCLUSION
LRINEC score may be used as an objective scoring system to predict the risk of amputation in patients with moderate to severe DFI in indoor clinical settings.
ETHICAL APPROVAL:
This study was approved by the ethical review board prior to the initiation of the research. (Approval No. CMH/ RWP/ IRB/1606-23).
PATIENTS’ CONSENT:
Participants of the study were briefed about the nature of study and informed consent was obtained from them to publish the data.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
AH, MTK: Study design, acquisition, analysis and data interpretation, drafting the manuscript, and critical review.
SMK: Study design, data interpretation, and critical review.
AT, MF, MAL: Conception, acquisition, data interpretation, and critical review.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. Diabetes Mellitus. In: Harrison’s Manual of Medicine, 20e [Internet]. New York, NY: McGraw-Hill Education; 2020 accessmedicine.mhmedical.com/content. aspx?aid=1167068671.
- Van Netten JJ, Bus SA, Apelqvist J, Chen P, Chuter V, Fitridge R, et al. I International Working Group on the Diabetic Foot. Definitions and criteria for diabetes-related foot disease (IWGDF 2023 update). Diabetes Metab Res Rev 2024; 40(3):e3654. doi: 10.1002/dmrr.3654.
- Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care 2020; 43(5):964-74. doi: 10.2337/dc19-1614.
- Lavery LA, Armstrong DG, Murdoch DP, Peters EJ, Lipsky BA. Validation of the infectious diseases society of America's diabetic foot infection classification system. Clin Infect Dis 2007; 44(4):562-5. doi: 10.1086/511036.
- Peters EJ, Childs MR, Wunderlich RP, Harkless LB, Arms-trong DG, Lavery LA. Functional status of persons with diabetes-related lower-extremity amputations. Diabetes Care 2001; 24(10):1799-804. doi: 10.2337/diacare.24.10.1799.
- Ndosi M, Wright-Hughes A, Brown S, Backhouse M, Lipsky BA, Bhogal M, et al. Prognosis of the infected diabetic foot ulcer: A 12-month prospective observational study. Diabet Med 2018; 35(1):78-88. doi: 10.1111/dme.13537.
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the eurodiale study. Diabetologia 2007; 50(1):18-25. doi: 10.1007/s00125-006- 0491-1.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32(7):1535-41. doi: 10.1097/01.ccm.0000129486.35458.7d.
- Monteiro-Soares M, Hamilton EJ, Russell DA, Srisawasdi G, Boyko EJ, Mills JL, et al. Guidelines on the classification of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev 2024; 40(3):e3648. doi: 10.1002/ dmrr.3648.
- O’Connell PR, McCaskie AW, Sayers RD, editors. Bailey & Love’s short practice of surgery. 27th edition. Boca Raton: CRC Press, Taylor & Francis Group. doi: org/10.1201/978 1315111087.
- Arifin WN. Sample size calculator (web) [Internet]. 2024 [cited 25 March 2024]. Available from: http://wnarifin. github.io
- Tuglo LS. Prevalence and determinants of lower extremity amputations among type I and type II diabetic patients: A multicenter-based study. Int Wound J 2023; 20(4):903-9. doi: 10.1111/iwj.13935.
- Campbell WC. Canale ST, Beaty JH. Campbell’s operative orthopaedics (11th ed). Philadelphia PA: Mosby/Elsevier (2008).
- Sen P, Demirdal T. Predictive ability of LRINEC score in the prediction of limb loss and mortality in diabetic foot infec-tion. Diagn Microbiol Infect Dis 2021; 100(1):115323. doi: 10.1016/j.diagmicrobio.2021.115323.
- Neeki MM, Dong F, Au C, Toy J, Khoshab N, Lee C, et al. Evaluating the laboratory risk indicator to differentiate cellulitis from necrotizing fasciitis in the emergency department. West J Emerg Med 2017; 18(4):684-89. doi: 10.5811/westjem.2017.3.33607.
- Hsiao CT, Chang CP, Huang TY, Chen YC, Fann WC. Prospective validation of the laboratory risk indicator for necrotizing fasciitis (LRINEC) score for necrotizing fasciitis of the extremities. PLoS One 2020; 15(1):e0227748. doi: 10.1371/journal.pone.0227748.
- Wu PH, Wu KH, Hsiao CT, Wu SR, Chang CP. Utility of modified Laboratory Risk Indicator for Necrotizing Fasciitis (MLRINEC) score in distinguishing necrotizing from non-necrotizing soft tissue infections. World J Emerg Surg 2021; 16(1):26. doi: 10.1186/s13017-021-00373-0.
- Walicka M, Raczyńska M, Marcinkowska K, Lisicka I, Czaicki A, Wierzba W, Franek E. Amputations of lower limb in subjects with diabetes mellitus: Reasons and 30-day mortality. J Diabetes Res 2021; 2021:8866126. doi: 10. 1155/2021/8866126.
- Narasimhan V, Ooi G, Weidlich S, Carson P. Laboratory risk indicator for necrotizing fasciitis score for early diagnosis of necrotizing fasciitis in darwin. ANZ J Surg 2018; 88(1-2): E45-9. doi: 10.1111/ans.13895.
- Vuorlaakso M, Kiiski J, Salonen T, Karppelin M, Helminen M, Kaartinen I. Major amputation profoundly increases mortality in patients with diabetic foot infection. Front Surg 2021; 8:655902. doi: 10.3389/fsurg.2021.655902.